Regulatory Workflows and Tools for the Gathering, Preparation, Validation and Packaging of Submissions to Regulatory Agencies
Xbiom™ manages data and documents through the lifecycle and the supply-chain of data from nonclinical studies and clinical trials while streamlining the interactions with various CROs, Specialty and Bio-Analytics labs, Bio-Metrics teams, data standards and terminology management, analytics, TFLs, report generation and packaging of submittable data into eCTD M4 and M5 folders. A series of workflows and tools for various transformations, conformance to standards, validation to standards and Technical Conformance Guides, and packaging under a 21 CFR Part 11 environment provide a compelling, cost-saving facility for sponsors and their regulatory or operations teams.
Xbiom workflows support planning of submissions to manage individual studies or trials while providing the specialized tools for packaging and validating Clinical SDTM, ADaM, and Nonclinical SEND datasets.
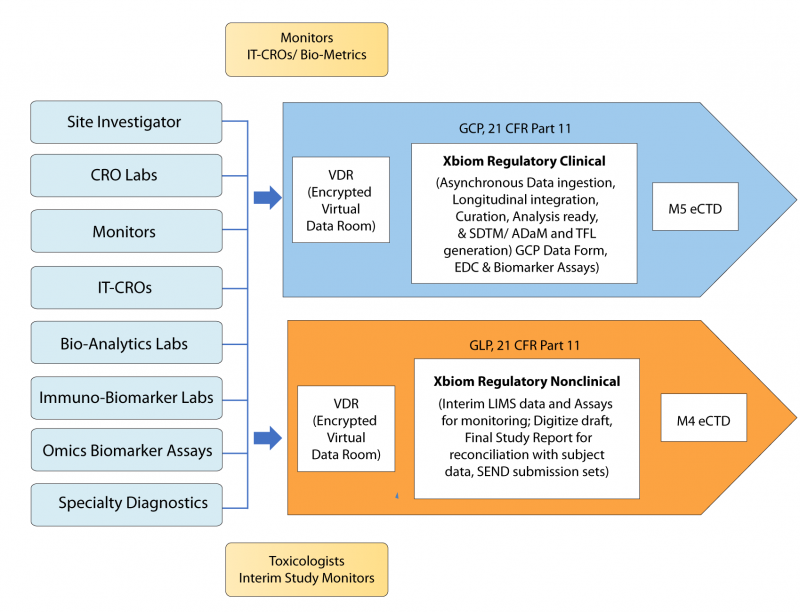
Clinical Regulatory Workflows, Tools and Metadata Governance
- Management and governance of CDISC and corporate standards driven metadata, data models and study level models from protocol to submission with workflows and role-based controls.
- Smart Transformation and machine learning tools for real-time EDC curation; longitudinal integration with Biomarker Assays for real-time monitoring of trial.
- Track & Identify Bio-Samples for Specialty Assays based on patient visits and translational needs.
- Warehouse of all regulatory data into a Universal Data Model with automated conversion to SDTM and ADaM generation.
- Statistical computing environment, analysis and visualization to generate TFLs for publishing and collaboration
- Plan submissions, manage studies with CROs and partners, package M5 for eCTD
Nonclinical Regulatory Workflows, Tools and Metadata Governance
- Interim Monitoring of ongoing studies longitudinally integrated, multiple LIMS extracts and assays.
- Statistical analysis of selected cohorts or groups with publishable TFLs.
- Digitize draft or final Study Report for use with Reconciler to generate curated study data and transformation to SEND.
- Warehouse of all regulatory and study data into a Universal Data Model with automated conversion to SEND IG with selected CTs.
- Generate Trial Design domains, TS.XPT, Define.xml, nSDRG with Xbiom tools.
- Plan submissions, manage studies with CROs and partners, package M4 for eCTD

