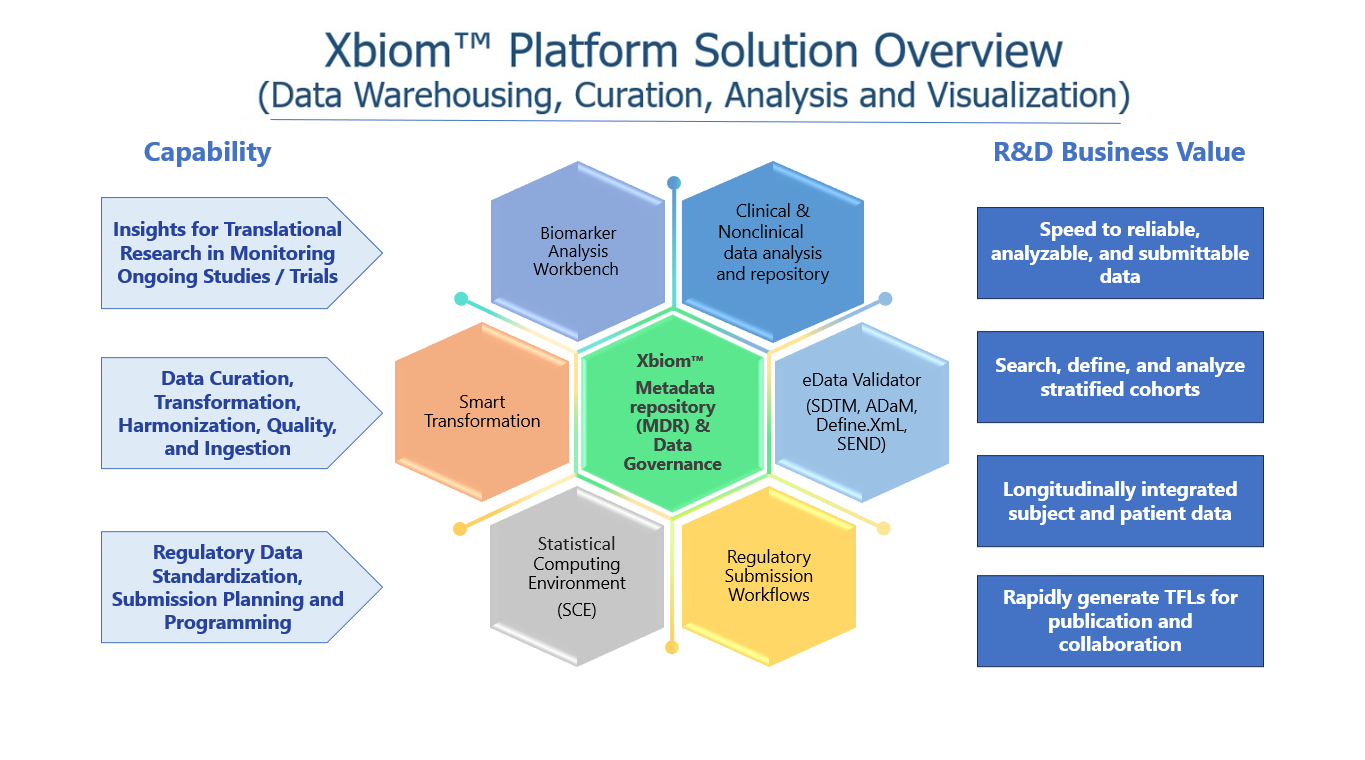
XBIOM™ is a robust, proven platform for data and information gathering, organizing, searching and building knowledge metadata and providing workflows, decision support tools, collaboration and publishing all in an encrypted secure, role based, scalable cloud-based facility.
Xbiom supports the Biotech and Biopharma industry with:
- Data curation, transformation to standards, and F.A.I.R. data management using smart transformers and machine intelligence.
- Universal Data Model for data, derived and extended metadata, and knowledge stores.
- Clinical and Nonclinical Insights from and study data, metadata and analysis.
- Regulatory, role-based workflows with 21 CFR Part 11 compliance, standards and metadata management, supported by ontologies and terminology management.
- Open Data Protocol (OData) and API enabled for access and exploitation by external enterprise applications.
- Extensible by adding custom modules on to common, secure infrastructure – such as Risk Monitoring and Stochastic Simulation.